AB INITIO
| Back |
|---|
All matter consists of atoms. An atom is the smallest stable particle of matter which can exist happily on its own. In a solid material like iron or glass or wood, the atoms are packed closely together. You can imagine them as just touching, as shown in figure 1. (You will have to use your imagination quite a lot when dealing with “fundamental particles”).
At any temperature above absolute zero, the atoms vibrate slightly and bump into one another and push themselves a little further apart. This is why substances expand as they get hotter. Further heating weakens the weak attraction between the atoms and they move almost independently as a liquid. Further heating further increases their speed of vibration and they eventually move completely independently and the substance become a gas.
At the end of the nineteenth century, atoms were discovered to consist of negatively charged and positively charged particles called electrons, (originally called corpuscles), and protons respectively. All negative charges were found to repel each other strongly and similarly for all positive charges. However, dissimilar charges were found to attract each other equally strongly. Originally atoms were thought to be just a mixture of the constituents, and this was known as the “Plum pudding model”. Each atom was assumed to contain an equal number of negative and positive charges and thus was electrically neutral. However, early in the twentieth century, Niels Bohr proposed that the electrons orbited the nucleus in fixed orbits rather as the earth and other planets orbit the sun. The “Bohr model” can be imagined as shown in figure 2.
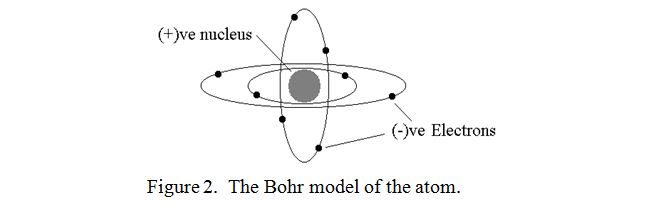
This is probably the most popular but not necessarily the most accurate model that we have of the atom. It can certainly be used to explain all the spectral lines which are emitted when gaseous atoms are energised as in a flame or in a fluorescent lamp, and it also explains many other phenomena. There are other models however, but we will stick with this one. (It is something of a paradox in particle physics that one has to use different mental models to explain different phenomena).


In a solid or liquid, the outermost orbiting electrons, (known as “Valence electrons”), often join the orbits of neighbouring atoms, performing a complicated and circuitous orbit embracing both or several nuclei. It is this sharing of orbits which binds each atom to its neighbour to form a solid or a liquid. In some substances, one or more electrons can escape from their orbit and wander off among the other atoms. These substances are called conductors and among them are all the metals. In other substances the electrons are tightly bound in their shared orbits and cannot easily escape and wander. These substances are called insulators.

Although I have quoted the masses and sizes of protons, neutrons, the nucleus and whole atoms, you don’t see many estimates of the size of an electron, for this is where the Bohr model comes unstuck. In other experiments, even where an electron is examined away from its parent atom, it behaves like a wave, and shows diffraction phenomena just like light or X-Rays, and it is not at all like a tiny compact mass or “corpuscle”. It still has a centre of mass and of charge whose position can be predicted with a certain probability, but it seems to be able to squeeze through two slits some distance apart at the same time. It is as if it were distributed in space with its charge and mass having a “sphere of influence” extending to infinity, with a calculable probability being ascribed to its being at any one place at any instant of time. In fact even larger particles such as atoms can show a wave nature. The electron can also spin on its own axis, generating its own small magnetic field. What all this means is that we really don’t understand matter at its deepest level. It now seems likely that the particles described above consist of even smaller particles, but there we must leave it for we are getting into the world of quantum dynamics. This is all rather disturbing for the deep thinking young scientist or radio amateur.
In spite of this uncertainty, we can predict the behaviour of electrons quite well, and we can build systems which use them, provided we use the appropriate “mental model”, which in most cases in electronics is the Bohr model. For example, we know that radiation is always produced by the acceleration of charge, so we can understand antennas. We know how to mix many substances so that electrons can build up internal electric fields in the substance to make diodes, transistors, photocells, etc. But before we get too smug, it is worth remembering that we don’t really know exactly what an electron is and probably never will. It is even possible that this level of understanding of particle physics is actually beyond human ability to build a satisfactory mental model using every-day concepts such as spheres, masses, particles and waves.
| Back |
|---|
